| Description: |
Tetracyclines generally act as bacteriostatic antibiotics. Broad spectrum antibiotics with activity against Gram-positive and Gram-negative bacteria. Resistance is widespread. The tetracyclines are a closely related group of antibiotics with comparable pharmacological properties but different pharmacokinetic characteristics.
|
||
| Mechanism of action: |
Tetracyclines inhibit bacterial protein synthesis. Their site of action is the bacerial ribosome - 30 S subunit, thereby preventing binding to those ribosomes of aminoacyl transfer-RNA. Active transport of the drug into the bacterial cell must occur in order for the drug to be effective. Doxycycline enters the cell by passive diffusion.
|
||
| Source: | ||
| Chlortetracycline | Streptomyces aureofaciens | |
| Oxytetracycline | Streptomyces rimosus | |
| Doxycycline |
Semisynthetic
|
|
|
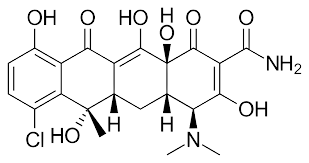
Chemical structure: |
The basic chemical structure of tetracyclines consists of a hydronaphthacene nucleus containing four hexacyclic fused rings. The chemical differences between the main members of the tetracycline family are in the substituents of three carbon atoms (C5, C6 and C7).
|
||
 |
|||||||||||||||||||||||
|
|||||||||||||||||||||||
| Indications: | Mycoplasma, Chlamydia, Pasteurella, Ornithobacterium rhinotracheale, Clostridium spp., Spirochetes and some protozoa. | ||
| Dosage: |
Chickens:
|
Turkeys:
|
Pigeons:
|
|
Oxytetracycline
|
50 mg/kg
|
50 mg/kg
|
ND
|
|
Doxycycline
|
ND
|
25 mg/kg
|
25 mg/kg PO
q12h ×3-5
days
|
| Drug-Drug Interctions: |
Co-administration of tetracyclines with antacids or other drugs containing divalent or trivalent cations, such as calcium, magnesium, iron or sodium content, is contraindicated. Tetracyclines form complexes with such cations, which are very poorly or not at all absorbed.
|
||
| Incompatibility: | Penicillins, cephalosporins. | ||
Pharmacokinetics: Variations in clinical efficacy of individual tetracyclines are attributable to differences in their pharmacokinetic properties rather than to differences in quantitative susceptibility of micro-organisms. The factor that underlies the pharmacokinetic properties of tetracyclines is lipid solubility; the degree of lipid solubility differs between individual tetracyclines.
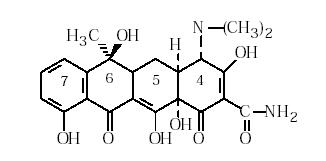
| Chemical structure: |
|
||||
| Animal species |
mg/kg bw
PO
|
t1/2
(Hr) |
Tmax |
F (%)
|
|
| Chicken (Broilers) | 10 | 30.59 ± 18.01 | 1.42 ± 0.86 |
30.54% ±
34 6.99
30.54 ± 6.99 |
|
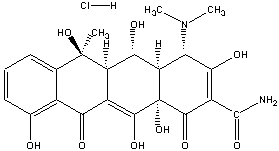
| Chemical structure: |
 |
||||
| Animal species |
mg/kg bw
PO
|
t1/2
(Hr) |
Tmax |
F (%)
|
|
| Turkey |
10
|
0.73±0.23
|
* 9.4
|
**47.6
|
|
| Chicken | 15 | 0.49 ± 0.38 | 2.85 ± 0.98 | 12.13 ± 4.56 | |
|
mg/kg bw
SC
|
|||||
| Chicken | 15 | 1.19 ± 0.52 | 3.95 ± 1.71 | 92.20 ± 10.53 | |
|
mg/kg bw
IM
|
|||||
| Chicken | 15 | 1.23 ± 0.36 | 2.6 ± 1.88 | 76.88 ± 12.90 | |
*Fed; **Fasted
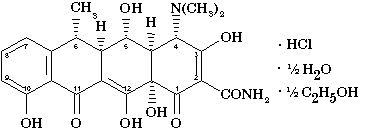
| Chemical structure: |
 |
||||
| Animal species |
Mg/kg bw
PO
|
t1/2
(Hr) |
Tmax
(Hr) |
F (%)
|
|
| Chickens |
20
|
6.03±0.45
|
0.35±0.02
|
41.33±2.02
|
|
| Turkeys 3 day-old |
25
|
10.6
|
*4.2
|
**4.7
|
* 40.0
** 83.7 |
| Turkeys 3 wks-old |
25
|
10.8
|
*5.3
|
**1.5
|
|
| Turkeys 6 wks-old |
25
|
7.9
|
*4.5
|
**2.8
|
|
| Turkeys 12 wks-old |
25
|
10.0
|
*7.5
|
**5.4
|
|
| Turkeys continous 4 day treatment |
25
|
ND
|
72±42.0
|
ND
|
|
*Fed; **Fasted
Drug Interactions: Tetracycline and tylosin exhibit synergistic activity against Pasteurella. In combination with polymyxin, tetracycline exhibits the synergistic activity because polymyxin increases the tetracycline uptake into the bacterial cell wall. Tetracycline antagonizes the action of beta-lactam antibiotics (bacteriostatic drugs inhibits the growth of bacteria and the bactericidal drugs act only upon growing bacteria).
Comments: Doxycycline is a second generation tetracycline mainly active against Gram-positive and Gram-negative aerobic and anaerobic bacteria. Doxycycline has low affinity for calcium and is relatively more stable in aqueous solution. It has a high relative liposolubility which readily compensates for the high protein binding. Doxycycline has several advanteges over other tetracycline analogues:
1. Absorption is almost complete
2. Tissue penetration is good
3. Elimination is slower
Various tetracyclines have been shown to accumulate in osseous tissues. High levels of tetracyclines can cause discoloration of egg shells (greenish-yellow) - Data indicates that a chlortetracycline level somewhere between .0625 and .1250 (1,135 grams per ton) percent of the diet is necessary to induce discoloration.