| Introduction: |
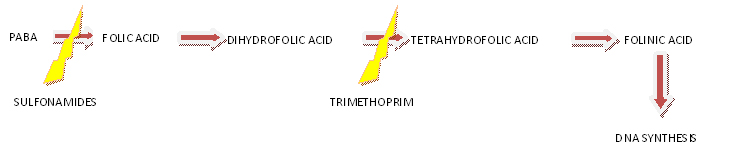
Tetrahydrofolic acid (THF) is a coenzyme in the synthesis of purine bases and thymidine. These are constituents of DNA and RNA and are required for cell growth and replication. Lack of THF leads to inhibition of cell proliferation.
|
| Description: |
Sulfonamides possess bacteriostatic activity against a broad spectrum of pathogens. Sulfonamides are produced by chemical synthesis.
|
| Mechanism of action: |
Sulfonamides structurally resemble p-aminobenzoic acid (PABA), a precursor in bacterial DHF synthesis. As false substrates, sulfonamides competitively inhibit utilization of PABA, and hence DHF synthesis. Because most bacteria cannot take up exogenous folate, they are depleted of DHF.
|
|
Trimethoprim:
|
Trimethoprim is a pyrimidine derivative used in combination with sulfonamides for enhanced antibacterial activity. Inhibits bacterial DHF reductase. Trimethoprim has bacteriostatic activity against a broad spectrum of pathogens. The antibacterial spectrum of trimethoprim is similar to that of sulfonamides, however, trimethoprim is 20- to 50-fold more potent.
|
| Indications: |
Gram-positive and gram-negative organisms as: Staphylococcus spp., Streptococcus spp., Pasteurella, Salmonella and E.coli.
|
| Dosage: |
Chickens:
|
Turkeys:
|
Pigeons:
|
|
--
|
- | - | - |
| - | - | - |
-
|
| - | - |
-
|
-
|
|
-
|
- | - | - |
|
-
|
- | - | - |
| Pharmacokinetics: |
Boulanger, Marine, et al. Poultry Science (2024): 104200.
|
||||||||||||||||||||
| Metabolism: |
The sulfa drugs are acetylated, primarily in the liver. The product is devoid of antimicrobial activity but retains the toxic potential to precipitate at neutral or acidic pH. This causes crystalluria and, therefore, potential damage to the kidney.
|
||||||||||||||||||||
| Toxicity: |
Suppression of bone marrow activity with resultant anaemia. Sulfonamides can cause decrease in egg shell thickness, soft-shelled eggs as well as rough surface (inhibition of the enzyme carbonic anhydrase).
|
||||||||||||||||||||
| Drug-Drug Interactions: |
Urine alkalinization increases the urinary excretion sulfonamides.
|
||||||||||||||||||||





